1° Fundamental Nature of Magnetism
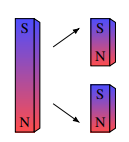
Unlike electric charges which can exist in isolation, magnetic poles always appear in pairs.
A magnet invariably has a north pole and a south pole, forming what is called a "magnetic dipole."
Magnetic Dipoles

If we try to isolate the magnetic poles by cutting a magnet, we simply end up with two smaller magnets, each retaining its two poles.
This division can be repeated indefinitely: each fragment, even at the atomic scale, always retains two poles.
Despite efforts to prove the existence of magnetic monopoles, none have ever been observed experimentally.
Microscopic Origin
Electrons can have two types of motions that generate magnetism:
Orbital angular momentum : the rotational motion of electrons around the nucleus
Spin : the electron rotates on itself, this is an effect of quantum physics.
Each type of movement is linked to a magnetic moment.
Orbital magnetic moment
This magnetic moment appears when a charge " q " moves with an orbital angular momentum " L ".
An electron orbiting the nucleus generates a circular current which generates a magnetic moment (field) whose vector is perpendicular to the plane of the orbit.
This orbital magnetic moment is called “(μL ) ⃗” and is:


e: charge of the electron
me: mass of the electron
L: orbital angular momentum
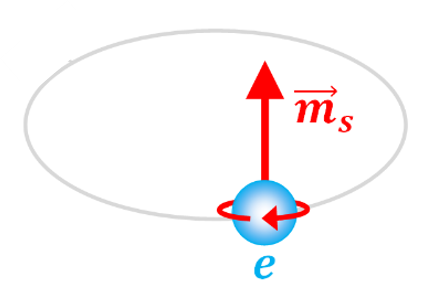
Spin magnetic moment
It is an intrinsic magnetic moment associated with the spin (intrinsic angular momentum) of the electron.
The electron behaves like a small magnet, and, even without an orbit, its spin generates a magnetic field.
The intrinsic spin of the electron, with two possible states: spin up or spin down.
This spin, although imaginary in terms of rotation, manifests a magnetic moment of spin “(μs ) ⃗”, which is worth:
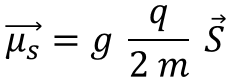
where g is the Landé factor (≈ 2 for the electron)

In quantum mechanics , the Landé factor is a dimensionless physical quantity that allows us to relate the magnetic moment to the angular momentum of a quantum state .
It is mainly used in the case of a non-zero spin particle .
It is named in honor of Alfred Landé who introduced it in 1921
Atomic magnetic moment
These different magnetic moments are combined (vector addition) to give the overall atomic magnetic moment.
These two vectors can be parallel (lower energy) or antiparallel (higher energy), which causes the fine structure of the energy levels via spin-orbit coupling.

Concrete applications
In the Zeeman effect , the total magnetic moment interacts with an external field, causing the energy levels to split.
In magnetic resonance , it is the spin moment (orientation ±½) which comes into play, controlled by external magnetic fields.
Magnetic materials : in certain materials, the moments combine and do not cancel each other out → ferromagnetism, paramagnetism, etc.
2° Classification of Magnetic Materials
Diamagnetic materials (silver, copper, water, gold, lead, zinc) have a zero atomic magnetic moment "µ" because all orbital and spin moments cancel each other out.
In this type of material, the external magnetic field creates an opposing induced magnetic field, causing a slight repulsive force.
Diamagnetic Materials

In paramagnetic materials (air, aluminum, magnesium, platinum), the atomic magnetic moments are not zero but are randomly oriented due to thermal agitation.
The overall magnetic moment therefore remains zero in the absence of an external field.
Paramagnetic Materials
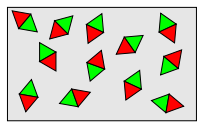
When an external magnetic field is applied, it exerts a moment of force on each magnetic dipole to align them with the field lines.
A balance is established between the aligning effect of the magnetic field and the disorienting effect of thermal agitation.
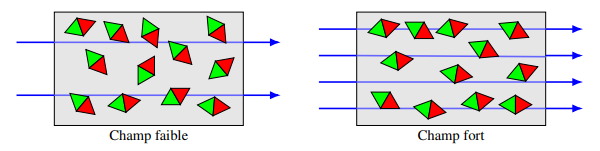
The partial alignment of the dipoles creates a net magnetic moment, and the material is attracted to regions of stronger field.

Ferromagnetic materials (cobalt, iron, nickel) exhibit exchange interactions between neighboring atoms that promote parallel alignment of magnetic moments.
This cooperation produces a very large atomic magnetic moment.
Ferromagnetic Materials

However, the orientation of the dipole moments is generally not uniform throughout the material.
The material is divided into regions called " Weiss domains ," in which all magnetic moments are aligned in the same direction.
" A Weiss domain is a microscopic region in a ferromagnetic material where the magnetic moments of the atoms are aligned in the same direction."

The orientation changes from one area to another, separated by "Bloch walls".
"Bloch walls are the transition zones between two adjacent Weiss domains where the direction of magnetization gradually changes from one orientation to another."
Under the influence of an external magnetic field, the favorably oriented domains expand to the detriment of the others, causing the progressive disappearance of the Bloch walls and the overall alignment of the material.

